Visit You tube channel for more MCQ's: https://www.youtube.com/channel/UCDI5O7VNvIby-Faq_ptLefw
Previous Year Questions (PYQ's)
1. From the following statements, (DEC 2016)
a) Hydrogen, Deuterium and Tritium differ in the number of protons.
b) Hydrogen, Deuterium and Tritium differ in the number of neutrons.
c) Both Deuterium and Tritium are radioactive and decay to hydrogen and deuterium respectively.
d) Tritium is radioactive and decays to helium.
e) Carbon-14 decays to Nitrogen-14.
f) Carbon-14 decays to Carbon-13.
Pick the combination with all correct statements.
A) a, b and f B) b, d and e
C) a, c and d D) c, e and f
Ans. B - b, d and e
Explanation: Hydrogen, deuterium and tritium are isotopes. Isotopes differ in the number of neutrons and hence statement "b" is correct. Deuterium is non-radioactive but tritium is radioactive. Tritium shows β decay and tritium decays to helium and hence statement "d" is also correct. Carbon-14 also shows β decay and carbon-14 decays to nitrogen-14 and hence statement "e" is also correct.
2. Which of the following pair represents isotones ? (Dec 2008)
A) H3, He4 B) N15, N14
C) Ba140, Th140 D) H1, H3
Ans. A - H3, He4
Explanation: Isotones are atoms having same number of neutrons but different number of protons. H3 have one proton and two neutrons. He4 have two protons and two neutrons. Both H3 and He4 have same number of neutrons and different number of protons.
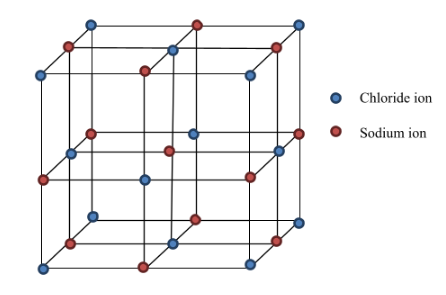
3. In crystalline NaCl, how many chloride ions surround each sodium ion ? (Dec 2022)
A) Four B) Six
C) Eight D) Ten
Ans. B - Six
Explanation: NaCl is an example of face centered cubic (fcc). It has atoms at each corner of the cube and six atoms at each face of the cube. You can observe the middle sodium ion (red) in the given diagram, chloride ions are in blue, one chloride ion is at top and one at bottom, two chloride ions are on the side, one chloride ion on the front and one on the back of sodium ion. Hence a total of six chloride ions surround each sodium ion.
4. Covalent bond formation between two atoms take place by (Dec 2008)A) Transfer of electrons from one atom to another.
B) One side sharing of electrons.
C) Electron Sharing by two electronegative atoms.
D) Affinity between two atoms.
Ans. C - Electron sharing by two electronegative atoms.
Explanation: Covalent bonds are formed when two atoms share electrons, typically between two non-metal atoms. This sharing allows both atoms to achieve a more stable electron configuration, usually by filling their outer electron shells. In covalent bonds, electrons are not transferred from one atom to another as in ionic bonds (option A), nor is there a one-sided sharing of electrons (option B) or affinity between two atoms (option D). Instead, covalent bonds are characterized by the mutual sharing of electrons between atoms with similar electronegativities.
5. The interaction energy between atom A and B is 400 kJ/mol. The type of interaction between them is (Dec 2013)
A) pi-pi B) covalent
C) ion-dipole D) hydrogen bond
Ans. B - covalent
Explanation: Strength of covalent bond can range from 200 to 450 kJ/mol, hence option B is correct
6. What would be approximate energy released on breaking H-H covalent bond ? (Dec 2008)
A) 4.32 X
105 J/mol B) 1 X 10-19
J/mol
C) 5 X 10-19
J/mol D) 8 X 1019
J/mol
Ans. A - 4.32 X 105 J/mol
Explanation: To calculate the energy released on breaking an H-H covalent bond, we need to consider the bond dissociation energy (BDE) of the H-H bond. The bond dissociation energy is the energy required to break a bond between two atoms in a molecule. For the H-H bond, the bond dissociation energy is approximately 436 kJ/mol.
Now, to convert this to joules per mole (J/mol), we use the conversion factor: 1 kJ/mol = 1000 J/mol. Therefore, the energy released on breaking the H-H covalent bond is:
436 kJ/mol * 1000 J/kJ = 436,000 J/mol
So, the closest option is: A) 4.32 X 10^5 J/mol
7. Arrange them according to decreasing order of strength. (June 2005)
A) sp3> sp2 > sp1 B) sp1 > sp2 >sp3
C) sp3> sp1 > sp2 D) sp1 > sp3 > sp2
Ans. B - sp1 > sp2 >sp3
Explanation: A bond with
sp1 hybridization is stronger than sp2 which is stronger
than sp3.
8. Half-life of radioactive material does not depend upon (Dec 2002)
A) Initial concentration of material
B) Decay constant.
C) Type of radioisotope.
D) Energy emission.
Ans. A - Initial concentration of material.
Explanation: The half life of radioactive material is independent of its initial concentration.
9. When an radioactive phosphorous is incorporated into DNA strand, the phosphodiester bond generally break after a short while because- (Dec 2004)
A) The bond strength of phosphorous decreases.
B) Phosphorous on radioactive decay converts into Sulphur, which has valence of two.
C) It attacks glycosidic bond.
D) Due to nucleophilic attack of 3'OH group
Ans. Phosphorous on radioactive decay converts into Sulphur, which has valence of two.
Explanation: When radioactive phosphorous (P32) is incorporated into a DNA strand, it replaces normal phosphorous in the DNA backbone. As P32 undergoes radioactive decay, it emits beta particles, which can damage the nearby sugar phosphate backbone of DNA. One common consequence of this damage is the breakage of the phosphodiester bond between adjacent nucleotides.
10. Given below are some physiochemical properties (column X) and their manifestations (column Y)
(Dec 2019)
|
X
|
Y
|
|
A. Pauling
electronegativity
|
i) Charge
separation
|
|
B. Isolated π
orbital
|
ii) Solvation
of atoms
|
|
C.
Aromaticity
|
iii)
Restricted rotation
|
|
D. Dielectric
|
iv) Planarity
of molecules
|
Which one of the following is the most appropriate match?
A) A-i, B-iv, C-ii, D-iii
B) A-iii, B-ii, C-iv, D-i
C) A-ii, B-iii, C-iv, D-i
D) A-iv, B-ii, C-i, D-iii
Ans. C) A-ii, B-iii, C-iv, D-i
Explanation:
Pauling electronegativity is a measure of an atom's ability
to attract electrons towards itself in a chemical bond. It indicates the
relative ability of an atom to attract electrons within a covalent bond.
When a highly electronegative atom is bonded to a less
electronegative atom, such as in a polar covalent bond, the highly
electronegative atom pulls the shared electrons closer to itself, creating a
partial negative charge (δ-) on its side of the bond and a partial positive
charge (δ+) on the less electronegative atom's side. This charge separation
contributes to the polarity of the molecule.
In the context of solvation, electronegativity plays a role
in determining how well an atom or molecule can be solvated by a solvent. When
an atom or molecule with a partial positive or negative charge (due to
differences in electronegativity) is introduced into a solvent, the solvent
molecules orient themselves around the solute molecules in a way that maximizes
interactions between opposite charges.
- Isolated
π orbitals: π orbitals are formed when p orbitals overlap sideways in
a covalent bond. In organic chemistry, molecules with conjugated systems
often have isolated π orbitals. Conjugated systems are arrangements of
alternating single and multiple bonds, allowing for delocalization of
electrons along the system.
- Restricted
rotation: In the context of molecules with isolated π orbitals,
restricted rotation refers to the limitation of movement around certain
bonds due to the presence of these π orbitals.
The planarity of aromatic molecules is intimately connected
to their aromaticity. Aromatic compounds tend to adopt planar geometries to
maximize the overlap of the p orbitals containing the delocalized π electrons.
This planarity is crucial for stabilizing the aromatic system through
conjugation, as it allows for efficient delocalization of electrons around the
ring.
The relationship between dielectric and charge separation
lies in the ability of dielectric materials to enhance or facilitate charge
separation. When an electric field is applied to a dielectric material, the
material polarizes, meaning its constituent atoms or molecules align in such a
way that the positive and negative charges become separated within the
material. This polarization effect results in an internal electric field that
opposes the external field.
11. The solubility of NaCl is greater in water than ethanol. What physical property of the solvent governs this difference? (June 2023)
1) Surface tension
2) Viscosity
3) Dielectric constant
4) Boiling point
Ans. 3 - Dielectric constant
Explanation: The solubility of a solute in a solvent depends on various
factors, including the nature of the solute and solvent, temperature, and
pressure. In the case of NaCl dissolving in water versus ethanol, the primary
factor at play is the dielectric constant of the solvent.
The dielectric constant (also known as relative
permittivity) measures a solvent's ability to reduce the electrostatic forces
between charged particles, such as ions. Water has a significantly higher
dielectric constant (around 80 at room temperature) compared to ethanol (around
24 at room temperature). This high dielectric constant of water means that it
can more effectively solvate the ions in NaCl, leading to greater solubility
compared to ethanol.
12. Maximum possible isomers for glucose are ( June 2009)
1) 4 2) 8
3) 16 4) 32
Ans. 3 - 16
Explanation: Glucose has four chiral carbons. So there are 24 or 16 possible
stereoisomers of this formula.
13. Glucose residues in amylose are linked by (Dec 2013)
1) β1→4 2) α1→4
3) α1→6 4) β1→6
Ans. 2) α1→4
Explanation: Glucose residues in amylose are linked by α1→4 glycosidic linkages.
14. Following are structures of stereoisomers of aldohexoses which differ in the stereochemistry
Based on the above structures, following information was given below: (June 2018)
A. D-glucose and D-mannose are epimers because they differ in the stereochemistry at C-2 position.
B. D-glucose and D-galactose are epimers because they differ in the stereochemistry at C-4 position.
C. D-mannose and D-glucose are epimers because they differ in the stereochemistry at C-3 position.
D. D-galactose and D-glucose are epimers because they differ in the stereochemistry at C-5 position.
Choose one of the correct combinations of above statements:
1) A and B 2) C and D
3) B,C and D 4) A and D
Ans. 1 - A and B
Explanation: Epimers specifically refers to stereoisomers that differ in the configuration of only one stereogenic center (chiral center) in a molecule. In the given figures it is clear that glucose and mannose are epimers which differ in the stereochemistry at C-2 position not at C-3. Glucose and galactose are epimers because they differ in the stereochemistry at C-4 position not at C-5.
15. A stoichiometric mixture of α and β anomers of D-glucose in water exhibits (Nov 2020)
1) net optical rotation proportional to the sum of the optical activities of each anomer.
2) No optical activity as the sign of optical rotation are opposite and they cancel each other.
3) No optical activity as the α and β anomers in the linear forms that are optically inactive.
4) no optical activity as they form a racemic mixture.
Ans. 1 - net optical rotation proportional to the sum of the optical activities of each anomer.
Explanation: When α and β anomers of D-glucose are dissolved in water,
they interconvert through mutarotation, reaching an equilibrium mixture where
both anomers are present. While the individual α and β anomers may have
opposite optical rotations, their concentrations are not necessarily equal in a
mixture. Therefore, the net optical rotation of the solution is proportional to
the sum of the optical activities of each anomer.
16. Milk sugar lactose, a disaccharide has linkage - (Dec 2003) 1) Glucose + Galactose β1➡4
2) Glucose + Glucose α1➡4
3) Glucose + Fructose α1➡6
4) Glucose + Galactose α1➡6
Ans. 1 - Glucose + Galactose β1➡4
Explanation: The correct linkage for milk sugar lactose, a disaccharide is Glucose + Galactose β1➡4
17. Sucrose does not have its anomeric forms while its hydrolyzed product glucose and fructose have anomers. The reason is (June 2007)
1) C1 of glucose and C1 of fructose are bonded in glycosidic linkage
2) C1 of glucose and C2 of fructose are bonded in glycosidic linkage
3) Sucrose is optically inactive
4) Sucrose lack chiral carbon centers
Ans. 4 - Sucrose lack chiral carbon centers
Explanation: Sucrose is composed of one molecule of glucose and one
molecule of fructose joined by a glycosidic bond between the anomeric carbon of
glucose (C1) and the hydroxyl group of fructose. Both glucose and fructose
individually have anomeric forms because they contain chiral carbon centers.
However, when they are linked in sucrose, the glycosidic bond removes the free
rotation around the linkage, preventing the formation of anomers in sucrose
itself.
18. In animals the carbohydrate energy reserve is in the form of (June 2002)
1) Inulin 2) Amylose
3) Cellulose 4) Glycogen
Ans. 4 - Glycogen
Explanation: Glycogen serves as the carbohydrate energy reserve in animals, primarily stored in liver and muscles.
19. Non-reducing disaccharide trehalose occurs in hemolymph of (June 2002)
1) Butterfly 2) Earthworm
3) Roundworm 4) Birds
Ans. 1 - Butterfly
Explanation: Trehalose occurs in hemolymph of many insects, hence option 1 is correct. Trehalose is indeed found in hemolymph of many arthropods, not just insects.
20. The structure of carbohydrate is shown as below. In cellulose polymer the bonding will be (Dec 2009)
1) α - 1,2 2) β - 1,4
3) α - 4,1 4) β - 2,4
Ans. 2 - β - 1,4
Explanation: Cellulose is a linear (unbranched) polymer consisting of β glucose units joined together by β - 1,4 glycosidic bonds.
Next SET:





Comments
Post a Comment